Matter is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other particles which have mass. A common way of defining matter is as anything that has mass and occupies volume. In practice however there is no single correct scientific meaning of "matter," as different fields use the term in different and sometimes incompatible ways.
For much of the history of the natural sciences people have contemplated the exact nature of matter. The idea that matter was built of discrete building blocks, the so-called particulate theory of matter, was first put forward by the Greek philosophers Leucippus and Democritus. Over time an increasingly fine structure for matter was discovered: objects are made from molecules, molecules consist of atoms, which in turn consist of interacting subatomic particles like protons and electrons.
PHASES OF MATTER
Matter can exist in four phases: solid, liquid, gas, and plasma, plus a few other extreme phases like critical fluids and degenerate gases.
Generally, as a solid is heated (or as pressure decreases), it will change to a liquid form, and will eventually become a gas. For example, ice (frozen water) melts into liquid water when it is heated. As the water boils, the water evaporates and becomes water vapor.
Sometimes, a solid will go directly from solid to gas - this is call subliming. An example of sublimation is dry ice, the solid (frozen) form of carbon dioxide, CO2, which turns into gaseous carbon dioxide at standard temperature and pressure - there is no liquid phase of CO2 at standard temperature and pressure.
Generally, as a solid is heated (or as pressure decreases), it will change to a liquid form, and will eventually become a gas. For example, ice (frozen water) melts into liquid water when it is heated. As the water boils, the water evaporates and becomes water vapor.
Sometimes, a solid will go directly from solid to gas - this is call subliming. An example of sublimation is dry ice, the solid (frozen) form of carbon dioxide, CO2, which turns into gaseous carbon dioxide at standard temperature and pressure - there is no liquid phase of CO2 at standard temperature and pressure.
Solid:
A solid is matter in which the molecules are very close together and cannot move around. Examples of solids include rocks, wood, and ice (frozen water). Liquid:
A liquid is matter in which the molecules are close together and move around slowly. Examples of liquids include drinking water, mercury at room temperature, and lava (molten rock).
Gas:
A gas is matter in which the molecules are widely separated, move around freely, and move at high speeds. Examples of gases include the gases we breathe (nitrogen, oxygen, and others), the helium in balloons, and steam (water vapor).
Plasma:
A plasma is a gas that is composed of free-floating ions (atoms stripped of some electrons - positively charged) and free electrons (negatively charged). A plasma conducts electrical currents. Plasma was discovered by William Crookes in 1879. There are many different types of plasmas. There is plasma in stars (including our Sun); the solar wind in our Solar System is made of plasma.
Supercritical Fluid:
A supercritical (or critical) fluid is a liquid/gas under extreme pressure. These supercritical fluids have unique characteristics, the density of a liquid and the mobility of a gas. Supercritical fluids exist deep inside some planets; for example, there is supercritical water deep inside the Earth.
Degenerate Gas:
A degenerate gas is one that is super-compressed and very dense. The molecules of this degenerate gas are virtually touching one another and the gas acts much like a solid. Unlike gases under normal conditions, the temperature in a degenerate gas does not depend on the pressure. These gases follow quantum mechanical laws.
Activity about Phases of Matter:
Phases of Matter Wheel Make a phases of matter wheel using this print-out; it consists of a base page together with a wheel that spins around. When you spin the wheel, three phases of matter appear: solid, liquid, and gas (plus an explanation and some examples of each). The student then writes down the phases of matter and one example of each. |
Before proceeding to the next topic, why don't we first learn a song? This song makes it easier for us to remember the phases/states of matter. This song is entitled STATES OF MATTER SONG.
Just as you use several adjectives to describe someone (color of hair or eyes, how tall or short, etc.) several properties, or characteristics, must be used in combination to adequately describe a kind of matter. Simply saying that something is a colorless liquid isn't enough to identify it as water. A lot of liquids are colorless, e.g. most alcohols and cyclohexane, as well as many solutions. More details are needed before one can zero in on the identity of a substance. Chemists will therefore, determine several properties, both chemical and physical, in order to characterize a particular sample of matter. The folowing chart shows the differences between the two kinds of properties, chemical and physical, as well as how the two kinds of physical properties, intensive and extensive, differ.

In a chemical change, bonds are broken and new bonds are formed between different atoms. This breaking and forming of bonds takes place when particles of the original materials collide with one another.
Whenever chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products are generated.
During chemical change, substances are changed into diffrent substances. Other examples of chemical changes are:
- Decomposition
- Neutralization
- Photosynthesis - a process in which carbon dioxide and water are changed into sugars by plants.
- Cracking heavy hydrocarbons to create lighter hydrocarbons.
- Cooking
- Oxidation
- Ripening
Evidence of a chemical change:
The following can indicate that a chemical change took place, although this evidence is not conclusive:
- Change of odor.
- Change of color.
- Change in temperature.
- Change of form.
- Light, heat, or sound is given off.
- Formation of gases, often appearing as bubbles.
- Formation of precipitate (insoluble particles).
- The decomposition of organic matter.
Physical change is a concept introduced to contrast with the concept of chemical change. A physical change is any change not involving a change in the substance's chemical identity. Matter undergoes chemical change when the composition of the substances changes: one or more substances combine or break up (as in a relationship) to form new substances. Physical changes occur when objects undergo a change that does not change their chemical nature.
An example of a physical change occurs when making a baseball bat. Wood is carefully crafted into a shape which will allow a batter to best apply force on the ball. Even though the wood has changed shape and therefore physical properties, the chemical nature of the wood has not been altered. The bat and the original piece of wood are still the same chemical substance.
ELEMENTS AND COMPOUNDS
"Don't Forget To Listen To This Song"
- Element - An element is a substance composed of the same type of atoms (e.g. gold Au, oxygen O2).
- Compound - A compound is a substance made of more than one type of atom2O, carbon dioxide CO2). (e.g. water H
- Molecule - A molecule is the smallest particle of either an element or a compound.
INERT OR NOBLE GASES
- Inert or Noble Gases are unreactive gases. They do not corrode nor react.
- Examples of Noble Gases are:
- He - Helium
- Ne - Neon
- Ar - Argon
- Kr - Krypton
- Xe - Xenon
- Rn - Radon
- The electron rings of these unreactive gases are full, therefore they become stable.
IONS (CHARGED ATOMS)
- When atoms react, they may either gain or lose electrons. Electrons have a negative charge. An atom gaining or losing electrons will get an overall charge.
- Positive Ions are atoms that have lost electrons (e.g. sodium Na1+)
- Negative Ions are atoms that have gained electrons (e.g. chlorine Cl1-)
- In chemical reactions, atoms tend to gain or lose electrons to resemble the electron numbers of the stable Noble Gases.
COVALENT AND IONIC COMPOUNDS
- Covalent Compound - a compound where electrons are shared between the atoms (e.g. carbon dioxide CO2)
- Ionic Compound - a compound formed from the attraction between positive and negative ions. For example in the ionic compound sodium chloride NaCl, the chlorine ion (Cl1-) gains one electron that was given by the sodium ion (Na1+).
COMMON ELEMENTS AND SYMBOLS TO LEARN
| Element Symbol | Element Name | Element Symbol | Element Name |
| H | Hydrogen | Mn | Manganese |
| He | Helium | Fe | Iron |
| Li | Lithium | Co | Cobalt |
| C | Carbon | Ni | Nickel |
| N | Nitrogen | Cu | Copper |
| O | Oxygen | Zn | Zinc |
| F | Fluorine | Br | Bromine |
| Ne | Neon | Ag | Silver |
| Na | Sodium | Sn | Tin |
| Mg | Magnesium | I | Iodine |
| Al | Aluminium | Ba | Barium |
| Si | Silicon | W | Tungsten |
| P | Phosphorus | Pt | Platinum |
| S | Sulphur / Sulfur | Au | Gold |
| Cl | Chlorine | Hg | Mercury |
| Ar | Argon | Pb | Lead |
| K | Potassium | Cr | Chromium |
| Ca | Calcium | Ti | Titanium |
| Pu | Plutonium | U | Uranium |
NAMING COMPOUNDS
| PREFIX OR SUFFIX | MEANING | EXAMPLE |
| Mono- | There is 1 atom of that type in that molecule | Carbon monoxide (CO) |
| Di- | There are 2 atoms of that type in the molecule | Carbon dioxide (CO2) |
| Bi- | Hydrogen is present in the molecule | Sodium bicarbonate(NaHCO3) |
| -ide | There are only 2 types of atoms present in the molecule | Lead oxide(PbO) |
| -ate | There are 3 or more types of atoms in the molecule, and 1 type is oxygen | Calcium carbonate(CaCO3) |
VALENCY TABLE
- Valency - the charge of an ion or radical which has either lost or gained electrons
- Note that metals lose electrons easily to become positive ions. This is why most metals are good conductors of electricity.
| 1+ | 2+ | 3+ | 1- | 2- | 3- |
| H 1+ | Mg 2+ | Al 3+ | F 1- | O 2-oxide | PO4 3-phosphate |
| Na 1+ | Ca 2+ | Fe 3+ferric | Cl 1- | S 2-sulphide | |
| Li 1+ | Cu 2+ | Br 1- | CO3 2-carbonate | ||
| K 1+ | Zn 2+ | OH 1-hydroxide | SO4 2-sulphate | ||
| Ag 1+ | Pb 2+ | NO3 1-nitrate | |||
| NH4 1+ammonium | Fe 2+ferrous | HCO3 1-bicarbonate |
WORKING OUT FORMULAE OF IONIC COMPOUNDS
(THE CROSS-OVER METHOD)
(THE CROSS-OVER METHOD)
- Step 1 - In the ionic compounds to be learnt in junior science, there are two parts to the ionic compound - the first is a positive ion (usually a metal e.g. Na1+) and the second is a negative ion (e.g. Cl1-).
- Step 2 - Using the valency table, write the two ions and their valencies.
- Step 3 - Now ignore the positive and negative signs. Cross-over the top valency number to the bottom of the other ion symbol. Do this for both.
- Step 4 - Write the completed formulae with those same numbers at the bottom.
- Step 5 - If the numbers on each part are the same (e.g. Na1 Cl1 or Mg2 O2), ignore them and rewrite the formulae without them (e.g. Na Cl or Mg O).
- Step 6 - Brackets may be used around radicals (groups of atoms that are charged e.g CO3).
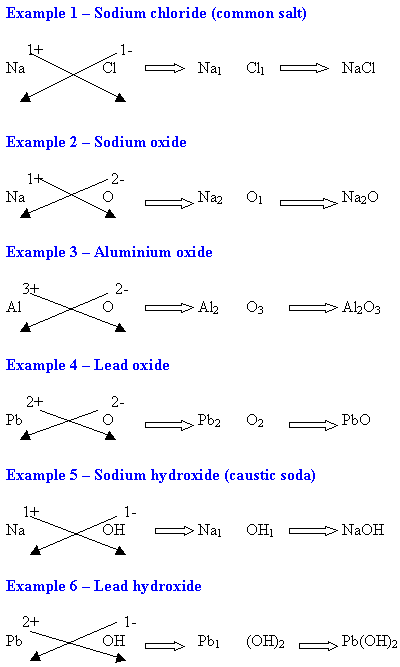
EXAMPLES OF CHEMICAL NAMES OF COMPOUNDS
| CHEMICAL FORMULA | CHEMICAL NAME |
| CO2 | carbon dioxide |
| CO | carbon monoxide |
| Na Cl | sodium chloride |
| Cu O | copper oxide |
| Ag Br | silver bromide |
| K I | potassium iodide |
| H Cl | hydrogen chloride (hydrochloric acid) |
| NH4 Cl | ammonium chloride |
| K OH | potassium hydroxide |
| Na OH | sodium hydroxide |
| Ca (OH)2 | calcium hydroxide |
| Ca S | calcium sulphide |
| Na NO3 | sodium nitrate |
| H NO3 | hydrogen nitrate (nitric acid) |
| Na HCO3 | sodium bicarbonate |
| Zn SO4 | zinc sulphate |
| Mg CO3 | magnesium carbonate |
| Ca SO4 | calcium sulphate |
| Cu CO3 | copper carbonate |
| Al PO4 | aluminium phosphate |
| Fe SO4 | iron sulphate |
| Fe CO3 | iron carbonate |
| NH4 NO3 | ammonium nitrate |
| NH4 HCO3 | ammonium bicarbonate |
| H2 SO4 | hydrogen sulphate (sulphuric acid) |
| Na2 SO4 | sodium sulphate |
| (NH4)2 CO3 | ammonium carbonate |
EXAMPLES OF NUMBERS AND TYPES OF ATOMS
IN VARIOUS ELEMENTS AND COMPOUNDS
IN VARIOUS ELEMENTS AND COMPOUNDS
| NAME OF SUBSTANCE | CHEMICAL FORMULA | ELEMENT OR COMPOUND | NUMBER AND TYPE OF ATOMS IN MOLECULE |
| Hydrogen | H2 | element | 2 hydrogen atoms |
| Carbon dioxide | CO2 | compound | 1 carbon atom 2 oxygen atoms |
| Water | H2O | compound | 2 hydrogen atoms 1 oxygen atom |
| Methane | CH4 | compound | 1 carbon atom 4 hydrogen atoms |
| Sodium hydroxide | NaOH | compound | 1 sodium atom 1oxygen atom 1 hydrogen atom |
| Calcium hydroxide | Ca(OH)2 | compound | 1 calcium atom 2 oxygen atoms 2 hydrogen atoms |
Mixtures and Compounds
Mixtures are heterogeneous forms of matter. Mixtures are composed of variable proportions of molecules and atoms.Compounds are homogeneous forms of matter. Their constituent elements (atoms and/or ions) are always present in fixed proportions (1:1 depicted here).

Examples of mixtures:
- soil
- ocean water and other solutions
- air
- the cytosol of a cell
Examples of compounds:
- water (H2O)
- table salt (NaCl)
- table sugar (C12H22O11)
Properties of Mixtures
- The composition of a mixture is variable.
- Each of its components retains its characteristic properties.
- Its components are easily separated.
Properties of Compounds
- The relative proportions of the elements in a compound are fixed.
- The components of a compound do not retain their individual properties. Both sodium and chlorine are poisonous; their compound, table salt - NaCl - is absolutely essential to life.
- It takes large inputs of energy to separate the components of a compound.
Separating the Components of a Mixture
 Most laboratory work in biology requires the use of techniques to separate the components of mixtures. This is done by exploiting some property that distinguishes the components, such as their relative
Most laboratory work in biology requires the use of techniques to separate the components of mixtures. This is done by exploiting some property that distinguishes the components, such as their relative- size
- density
- solubility
- electrical charge
Dialysis
Dialysis is the separation of small solute molecules or ions (e.g., glucose, Na+, Cl-) from macromolecules (e.g., starch) by virtue of their differing rates of diffusion through a differentially permeable membrane.
An example:
Cellophane is perforated with tiny pores that permit ions and small molecules to pass through but exclude molecules with molecular weights greater than about 12,000. If we fill a piece of cellophane tubing with a mixture of starch and sugar and place it in pure water, the sugar molecules (red dots) will diffuse out into the water until equilibrium is reached; that is, until their concentration is equal on both sides of the membrane. Because of their large size, all the starch (blue disks) will be retained within the tubing.Chromatography
Chromatography is the term used for several techniques for separating the components of a mixture. Follow the links below for examples.| Link to a description of paper chromatography, where the molecules are separated by size and solubility |
| Link to a description of exclusion chromatography, where the molecules in a mixture are separated by size. |
| Link to a description of affinity chromatography, where molecules are separated on the basis of their attraction to material in the chromatographic column. |
Electrophoresis
Electrophoresis uses a direct electric current to separate the components of a mixture by the differing electrical charge.| Link to a description of how the proteins in blood serum are separated by electrophoresis. |
Pure Substances
Some of the pure substances isolated from mixtures cannot be further broken down. Oxygen (O2) is an example. It is one of the elements; the fundamental building blocks of matter.| Link to discussion of elements. |
Most pure substances are compounds. Table salt, sodium chloride (NaCl), is an example; water (H2O) is another. If we pass an electrical current through molten NaCl, two new substances will be formed:
- sodium, a shiny metal so reactive that it must be stored out of contact with the air
- chlorine, a yellowish poisonous gas.
- The decomposition of NaCl required a large input of energy. This is because of the strength of the ionic bonds holding the Na and Cl atoms together.
- The ratio of the weights of the two products are always 23 parts of sodium to 35.5 parts of chlorine. This reflects:
- the invariance of the ratio (1:1 in this case) of the number of atoms in a compound
Link to discussion of valence. - the relative weights (23:35.5) of the atoms in table salt.
- The properties of the components of the compound are not the same as those of the compound itself. Both sodium and chlorine are hazardous to life; their compound, sodium chloride, is a vital ingredient of all animal diets.
